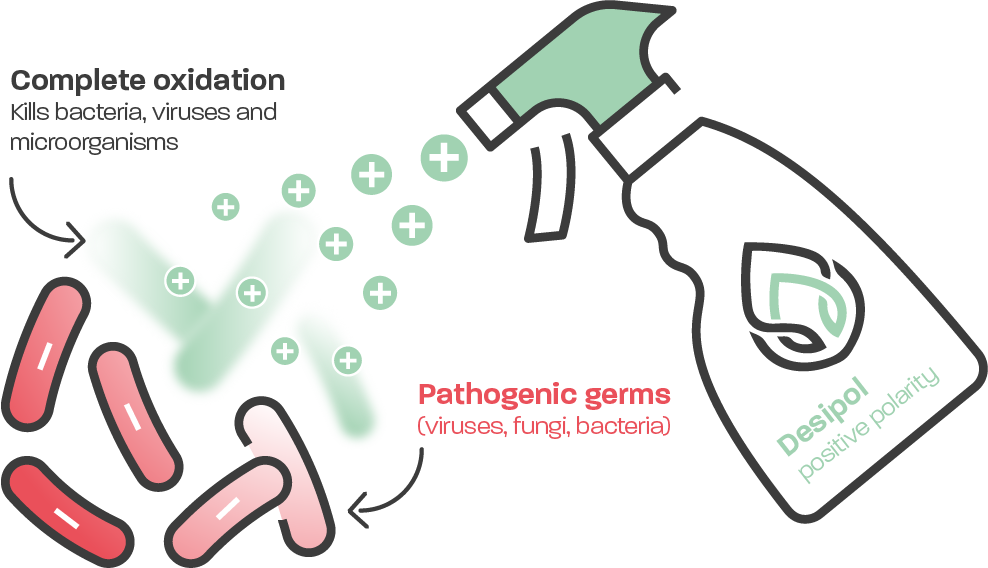
How Desipol® works
Desipol® discharges foreign bacteria, viruses and microorganisms and renders them harmless, with absolutely no side effects for humans, animals and plants. The disinfectant is made from drinking water. An electrolysis process uses electrical voltages and currents to break water down into its components, breaking the molecular hydrogen bonds in the process. Desipol® is then able to penetrate cell walls by osmosis thanks to the resulting high gas pressure.
A charge exchange occurs in the pathogenic germs which cause disease – the unequal polarity of the germ cell attracts the charged micro-fine gas bubbles in the disinfectant. Once they have penetrated the cell wall by osmosis, they neutralise the static charge of the membrane causing it to collapse. This is how the germ cell is destroyed and rendered harmless from within.
The following results using DESIPOL were calculated based on numerous laboratory reports, including some by the internationally recognised Biolytix AG, CH-4108 Witterswil:
The test product Desipol® was tested in a bactericidal efficacy test according to SN EN ISO1276:2020 after constant storage at 20°C and compared to a temporary cooling effect (2 variants: 20-hour storage at -20°C and 20-hour storage at -80°C prior to testing).
Test conditions:
Test concentration of test substances: 100%
Low exposure test condition: in the presence of 0. 3 g bovine serum albumin/ litre
Time of action test substance: 3 min, temperature: 25°C
Test germ: Escherichia coli DSM 682, ATCC 10 536
Composition Desipol®: drinking water 99. 96%, chlorine < 0. 1% (NaOCl 0. 035%, ClO2 0. 0011%, NaClO3 0. 0015%), ozone 0. 00 002%
| Test approach | Escherichia coli [KBE/ml] | Log-reduction (calculated), decimal-logarithmic | Evaluation for disinfecting efficacy according to SN EN1276:2020 (≥Log 5) |
|---|---|---|---|
| Control test approach: without addition of Desipol® | 1,8 x 10⁷ | - | - |
| Test approach: with addition of Desipol®, 20°C | 4,3 x 10¹ | > 5 | disinfectant efficacy (≥Log 5) |
| Test approach: with addition of Desipol®, after 20h storage at -20°C | 3,2 x 10⁵ | < 2 | no disinfectant efficacy |
Test approach: with addition of Desipol®, after 20h storage at -80°C | 5,8 x 10⁶ | < 1 | no disinfectant efficacy |
Evaluation
Desipol®, which had been stored at 20°C since receipt of the sample according to the manufacturer's instructions, showed a germ reduction of the test strain Escherichia coli DSM 682 by more than 5 log levels, which must be fulfilled according to EN1276 for chemical disinfectants. In contrast, Desipol®, which had first been stored at room temperature after receipt of the sample and then at -20°C or -80°C for 20 hours prior to the efficacy test, no longer showed any corresponding reduction in germs in the test strain. After exposure to cold, the test sample no longer met the requirements for an effective disinfectant.
This report has been submitted electronically by Dr. Diana Hormisch, Head of Microbiology, Food & Agro Diagnostics, and is valid without signature.

